瑞德西韦数据出来了
乌贼的马甲 • • 13515 次浏览简单概括下来就是对50%病患有效,其中一半两周内出院,今晚美股要大涨了。
某国最先爆发疫情,最先拿到药,最先展开盲测,最后却因病人不够宣告此药无效,真的是一声叹息。
-
#1
不带中医玩,神药说你无效就无效就怕以后有需要的时候,又厚着脸皮中西医结合了
-
#2
编继续编 没有细节数据的新闻不是好新闻
-
乌贼的马甲 楼主#3
想看细节自己去找啊这种事想编也编不出来啊
-
#4
呵呵 没有数据的文章多好编
-
#5
这个50%不是很理解假如对照组吃糖豆的60%有效,是不是糖豆更神呢?
-
#6
看新闻没觉得特别有效啊“It’s not going to be a cure, but it is going to be a drug potentially that if you use it particularly early in the course of the disease ... it could reduce their chances of having a really bad outcome,”
-
#7
是啊,没有对照组,很难说多有效然后5天和10天用药没差。这句话好了看就是5天就有效了,坏了看就是后面用药也没用了。
-
#8
有没有效,资本市场说明了一切神药有效的消息能促进吉利德以及全球股市上涨。而中药有效的消息,最多是莲花清瘟那个股票涨,其余的都没反应。
-
乌贼的马甲 楼主#9
我也没强逼着让你信不是?
新闻出来了 大家都有自己解读,信不信都是自己的事儿。
有人信了买了股票赚一笔,有人不信在那酸葡萄酸莲花清,这都是个人的选择,列出再多的数据也没用,你说是不是?
-
乌贼的马甲 楼主#10
对啊,有没有效时间会说明一切
这是Gilead自己发布的数据,之前好几个第三方trial也给出类似结果,当然不包括泄漏世卫数据的那家,后续更详细的数据马上会出来,FDA已经开始在程序上把瑞德西韦列为治疗新冠的专用药了。
我们应该为有有效药物的出现感到高兴,可是有些人看不得这些。幸好我们是在小坡,FDA批准后这边应该很快也会有了。
-
#11
当时双黄连股票还暴涨呢安慰作用
-
#12
世卫泄露的也是吉利德自己给的只是泄得早了点。套路啊
-
乌贼的马甲 楼主#13
吉利德上报自己的数据给WHO
然后被WHO不知道哪位大人给mistakenly post 到WHO的网站。这怎么看也不是吉利德自己要泄漏的吧
-
#14
建议了解一下吉利德公司先这种公司,和连花清瘟以及双黄连根本不是一个层面的。这也是为啥中药炒作只能炒个股,而吉利德能带的动美股甚至全球股市的大盘。
-
#15
前面中国也说了,效果肯定是有的,但不是特效,初期有效。跟WHO 现在的说法不是挺一致的吗。
说实话比我预期值要低...我一直希望中国说的“不是特效药”是骗人的。
在没有对照组的情况下,5天用药出院60%多,10天用药出院50%。对比来看不知道算不算好消息。
希望快点出特效药吧。
中西结合我觉得也有道理啊,中医调节安抚免疫系统(当然西医也可以,但我觉得中医在调理上还是有用的),西医控制病毒增殖。这个病后期完全是免疫系统乱了,不是仅仅是病毒本身的问题。
中医西医一定要非黑即白吗? -
#16
这个公司怎么了?从上市之后好像股价一直跌来着,直到这次covid
-
#17
特效谈不上如果真的是特效,大统领也不会强推消毒剂了^=^
-
#18
我特么算是服了一个医疗效果的讨论,居然用股市涨跌来论证。
-
#19
数据说话,各自解读, 形势需要
In that 397-patient trial, Gilead said 62% of patients treated early with remdesivir were discharged from the hospital, compared with 49% of patients who were treated later in the course of the infection.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, said partial results from its 1,063-patient trial show that hospitalized COVID-19 patients given remdesivir recovered in 11 days, compared to 15 days for patients given a placebo.
The study showed a trend toward better survival for remdesivir - 8% of patients given the drug died compared with 11.6% in the placebo group - but the difference was not statistically significant so may not be due to Gilead’s drug.
-
#20
Gilead server down原文找不到
新闻报告里前几次报告的死亡率,副作用都没提到 -
#21
都散了吧,这货就一爱崇洋舔美的。大部分确诊的不用任何药物,身体自行抗体免疫自我痊愈,去看看新加坡那一万多人有哪个是用药的?有哪个死的?按你这说法我随便开发个大力神丸给这新加坡一万多人吃,反正没人死,然后每天都有人出院痊愈,再对外公布说咱家的大力神丸有多牛逼赚个盆满钵满。呵呵
-
乌贼的马甲 楼主#22
你这货又是哪一路的?
随便给别人扣帽子的人是什么货?
这个数据是在重症患者群的trial,杠之前请认真读一下。
-
#23
-
#24
显然不符合常规的报告对照组呢?
-
#25
4月23号的报告
According to the summary of the China study, remdesivir was “not associated with a difference in time to clinical improvement” compared to a standard of care control. After one month, it appeared 13.9% of the remdesivir patients had died compared to 12.8% of patients in the control arm. The difference was not statistically significant.
-
乌贼的马甲 楼主#26
最新的数据不是双盲,是根据clinical trial得出来的如果被分到安慰剂那组,死亡率还是蛮高的
-
乌贼的马甲 楼主#27
我猜测现在的重症患者没几个敢参加双盲的如果被分到安慰剂那组,死亡率还是挺高的
-
#28
你弄错了,不是这个试验一共三个试验发布。gilead发布的是5天和10天的对比,没有显著差别,就是用10天的药是浪费的,只要用5天的药就够了,可以节省些药用来治更多的人。
还有一个证明有效的大规模双盲对比试验是这个
https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
最后,那个由于入组人数不足提前终止的中日友好医院的双盲对比验,得出的结论,在此次临床试验中,瑞德西韦对重症新冠肺炎患者临床症状没有明显改善效果,其有效性仍有待研究。也就是之前WHO提前泄露的那个。
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext
最后说一下,好像世界上不存在针对病毒的特效药,不需要反复重复强调“不是特效”,因为这本来就是预期中的。大规模双盲对比试验证明死亡率没有改善,但可以减少30%的住院时间,可以节省很多床位。但是,大部分国家通过隔离和扩容,床位不足问题目前已经不严重了。了胜于无吧。 -
#29
我相信数据是真实的换了个积极的说法而已
但是3期报告这样不是科学报告可以接受的
new release 没问题,随便怎么解释。
Gilead的三期是双盲设计的
https://www.businessinsider.sg/coronavirus-treatment-data-on-gilead-remdesivir-who-2020-4?r=US&IR=T
The trial ended up including 237 participants, with 158 receiving remdesivir and 79 receiving a placebo. It was designed to enroll 453 people.
The summary said that about 14% of those taking remdesivir had died after a month, compared with about 13% of those in the placebo group, -
#30
读了一下,这个就是最新的 5-10天比较
其他的完全没有涉及 -
#31
另外两个也是最新的April 29, 2020认真读一读吧
-
乌贼的马甲 楼主#32
你帮某些懒人把数据链接都找全了
对病毒没有特效药这点我同意,我觉得瑞德西韦之于新冠有点像tamiflu之于流感,做不到药到病除,但是能有效的减缓病症和缩短患病时间。
-
#33
赞这个层主赞这个层主
连花清瘟胶囊作为我们祖国文化精粹的代表在这次瘟疫中遭到了强烈的打压 -
#34
自己和自己比,5天和10天比按我的理解,只能(要)用5天, 10天反而更差, 对不对呢?
-
#35
读吧看这个药到底怎么样?
有效还是”需要“? -
#36
火了,全文再此Gilead Announces Results From Phase 3 Trial of Investigational Antiviral Remdesivir in Patients With Severe COVID-19April 29, 2020 at 8:35 AM EDTPDF Version
-- Study Demonstrates Similar Efficacy with 5- and 10-Day Dosing Durations of Remdesivir --
FOSTER CITY, Calif.--(BUSINESS WIRE)--Apr. 29, 2020--Gilead Sciences, Inc. (Nasdaq: GILD) today announced topline results from the open-label, Phase 3 SIMPLE trial evaluating 5-day and 10-day dosing durations of the investigational antiviral remdesivir in hospitalized patients with severe manifestations of COVID-19 disease. The study demonstrated that patients receiving a 10-day treatment course of remdesivir achieved similar improvement in clinical status compared with those taking a 5-day treatment course (Odds Ratio: 0.75 [95% CI 0.51 – 1.12] on Day 14). No new safety signals were identified with remdesivir across either treatment group. Gilead plans to submit the full data for publication in a peer-reviewed journal in the coming weeks.
“Unlike traditional drug development, we are attempting to evaluate an investigational agent alongside an evolving global pandemic. Multiple concurrent studies are helping inform whether remdesivir is a safe and effective treatment for COVID-19 and how to best utilize the drug,” said Merdad Parsey, MD, PhD, Chief Medical Officer, Gilead Sciences. “These study results complement data from the placebo-controlled study of remdesivir conducted by the National Institute for Allergy and Infectious Diseases and help to determine the optimal duration of treatment with remdesivir. The study demonstrates the potential for some patients to be treated with a 5-day regimen, which could significantly expand the number of patients who could be treated with our current supply of remdesivir. This is particularly important in the setting of a pandemic, to help hospitals and healthcare workers treat more patients in urgent need of care.”
Remdesivir is not yet licensed or approved anywhere globally and has not yet been demonstrated to be safe or effective for the treatment of COVID-19. This study sought to determine whether a shorter, 5-day course of remdesivir would achieve similar efficacy results as the 10-day treatment regimen used in multiple ongoing studies of remdesivir. Secondary objectives included rates of adverse events and additional measures of clinical response in both treatment groups. Patients were required to have evidence of pneumonia and reduced oxygen levels that did not require mechanical ventilation at the time of study entry. Clinical improvement was defined as an improvement of two or more points from baseline on a predefined seven-point scale, ranging from hospital discharge to increasing levels of oxygen support to death. Patients achieved clinical recovery if they no longer required oxygen support and medical care or were discharged from the hospital.
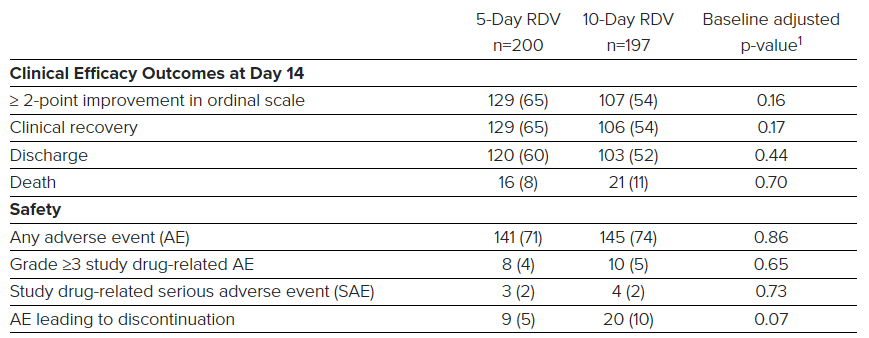
In this study, the time to clinical improvement for 50 percent of patients was 10 days in the 5-day treatment group and 11 days in the 10-day treatment group. More than half of patients in both treatment groups were discharged from the hospital by Day 14 (5-day: 60.0%, n=120/200 vs.10-day: 52.3% n=103/197; p=0.14). At Day 14, 64.5 percent (n=129/200) of patients in the 5-day treatment group and 53.8 percent (n=106/197) of patients in the 10-day treatment group achieved clinical recovery.
Clinical outcomes varied by geography. Outside of Italy, the overall mortality rate at Day 14 was 7 percent (n=23/320) across both treatment groups, with 64 percent (n=205/320) of patients experiencing clinical improvement at Day 14 and 61 percent (n=196/320) of patients discharged from the hospital.
Impact of Earlier Treatment
In an exploratory analysis, patients in the study who received remdesivir within 10 days of symptom onset had improved outcomes compared with those treated after more than 10 days of symptoms. Pooling data across treatment arms, by Day 14, 62 percent of patients treated early were able to be discharged from the hospital, compared with 49 percent of patients who were treated late.
“These data are encouraging as they indicate that patients who received a shorter, 5-day course of remdesivir experienced similar clinical improvement as patients who received a 10-day treatment course,” said Aruna Subramanian, MD, Clinical Professor of Medicine, Chief, Immunocompromised Host Infectious Diseases, Stanford University School of Medicine, and one of the lead investigators of the study. “While additional data are still needed, these results help to bring a clearer understanding of how treatment with remdesivir may be optimized, if proven safe and effective.”
Remdesivir was generally well-tolerated in both the 5-day and 10-day treatment groups. The most common adverse events occurring in more than 10 percent of patients in either group were nausea (5-day: 10.0%, n=20/200 vs. 10-day: 8.6%, n=17/197) and acute respiratory failure (5-day: 6.0%, n=12/200 vs. 10-day: 10.7%, n= 21/197). Grade 3 or higher liver enzyme (ALT) elevations occurred in 7.3 percent (n=28/385) of patients, with 3.0 percent (n=12/397) of patients discontinuing remdesivir treatment due to elevated liver tests.
Key efficacy and safety results from the study are included in the table below.
5-Day RDV
10-Day RDV
Baseline adjusted
n=200
n=197
p-value1
Clinical Efficacy Outcomes at Day 14
≥ 2-point improvement in ordinal scale
129 (65)
107 (54)
0.16
Clinical recovery
129 (65)
106 (54)
0.17
Discharge
120 (60)
103 (52)
0.44
Death
16 (8)
21 (11)
0.70
Safety
Any adverse event (AE)
141 (71)
145 (74)
0.86
Grade ≥3 study drug-related AE
8 (4)
10 (5)
0.65
Study drug-related serious adverse event (SAE)
3 (2)
4 (2)
0.73
AE leading to discontinuation
9 (5)
20 (10)
0.07
1Adjusted for baseline clinical status
About the SIMPLE Trials
Gilead initiated two randomized, open-label, multi-center Phase 3 clinical trials for remdesivir, the SIMPLE studies, in countries with high prevalence of COVID-19 infection.
The first SIMPLE trial is evaluating the safety and efficacy of 5-day and 10-day dosing regimens of remdesivir in hospitalized patients with severe manifestations of COVID-19. The initial phase of the study randomized 397 patients in a 1:1 ratio to receive remdesivir 200 mg on the first day, followed by remdesivir 100 mg each day until day 5 or 10, administered intravenously, in addition to standard of care. An expansion phase of the study was recently added and will enroll an additional 5,600 patients, including patients on mechanical ventilation. The study is being conducted at 180 trial sites around the world, including sites in the United States, China, France, Germany, Hong Kong, Italy, Japan, Korea, the Netherlands, Singapore, Spain, Sweden, Switzerland, Taiwan and the United Kingdom.
A second SIMPLE trial is evaluating the safety and efficacy of 5-day and 10-day dosing durations of remdesivir administered intravenously in patients with moderate manifestations of COVID-19, compared with standard of care. The results from the first 600 patients of this study are expected at the end of May.
About Remdesivir
Remdesivir is an investigational nucleotide analog with broad-spectrum antiviral activity both in vitro and in vivo in animal models against multiple emerging viral pathogens, including Ebola, Marburg, MERS and SARS. In vitro testing conducted by Gilead has demonstrated that remdesivir is active against the virus that causes COVID-19. The safety and efficacy of remdesivir for the treatment of COVID-19 are being evaluated in multiple ongoing Phase 3 clinical trials.
About Gilead Sciences
Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead has operations in more than 35 countries worldwide, with headquarters in Foster City, California.
For more information on Gilead’s response to the coronavirus outbreak please visit the company’s dedicated page: https://www.gilead.com/purpose/advancing-global-health/covid-19.
Forward-Looking Statement
This press release includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors. Remdesivir is an investigational agent that has not been licensed or approved anywhere globally, and it has not been demonstrated to be safe or effective for any use, including for the treatment of COVID-19. There is the possibility of unfavorable results from ongoing and additional clinical trials involving remdesivir and the possibility that Gilead and other parties may be unable to complete one or more of such trials in the currently anticipated timelines or at all. Further, it is possible that Gilead may make a strategic decision to discontinue development of remdesivir or that FDA and other regulatory agencies may not approve remdesivir, and any marketing approvals, if granted, may have significant limitations on its use. As a result, remdesivir may never be successfully commercialized. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in Gilead’s periodic reports filed with the U.S. Securities and Exchange Commission, including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements.
-
#37
只能说五天non inferior 或者10天 non superior
-
#38
比如tamiflu 对付流感这种。万众期待的就是特效药啊
-
乌贼的马甲 楼主#39
【其它话题】瑞德西韦数据出来了
简单概括下来就是对50%病患有效,其中一半两周内出院,今晚美股要大涨了。
某国最先爆发疫情,最先拿到药,最先展开盲测,最后却因病人不够宣告此药无效,真的是一声叹息。
该帖荣获当日十大第1,奖励楼主25分以及37狮城帮币,时间:2020-04-30 22:00:01。
-
#40
同一天的柳叶刀 (4月29日)双盲,用药组死亡稍高(统计无差别)
-
#41
NIH使用金标准证明有效金标准:大规模(1063 patients),双盲
效果:31% faster time to recovery (p<0.001)
有以上几个字数,“有效”已经基本是没有争议了。等完整数据公布,FDA必定会加速批准,成为唯一一个通过双盲3期临床试验的治疗COVID-19药物。 -
#42
你说的我同意,FDA本来就批了 -孤儿药的,(既然双盲,Gilead怎么不公布对照组数据,而是发正面新闻稿-还是公关意味大于科学证据吧)
国内这么认为:
权威医学期刊《柳叶刀》4月29日发表了由瑞德西韦中国临床试验负责人、中日友好医院副院长曹彬教授和北京协和医学院院校长王辰院士所写的,关于全球首项关于吉利德在研抗病毒药物瑞德西韦的随机双盲对照组、多中心临床试验论文,显示瑞德西韦对于重症患者无显著疗效。
同一天吉利德科学公司发布其针对397名患者的开放标签临床III期试验结果显示,瑞德西韦早期治疗效果显著,超过一半患者在两周内出院。
美国国家过敏与传染病研究所(NIAID)所长安东尼·福奇当天表示,瑞德西韦临床试验数据积极,显著缩短了病人恢复的时间,将成为新冠治疗的新标准。
针对中美临床试验为何出现截然相反的结果,瑞德西韦中国临床试验负责人曹彬教授向第一财经记者独家表示,研究终点不同导致了结果的差异,中国的研究设计更严格。
中国临床试验显示瑞德西韦对重症无效
《柳叶刀》最新发表的瑞德西韦首项临床试验论文的通讯作者为中日友好医院副院长曹彬教授和北京协和医学院院校长王辰院士。在对2月6日至3月12日期间招募的237名来自武汉10家医院的重症患者进行临床试验后,研究作者通过预先确定的临床试验主要终点得出结论,瑞德西韦用药与临床症状的改善关系不大。
从28天死亡率来看,瑞德西韦组和对照组分别为14%和13%;两组的副作用比例分别为66%和64%。此外,临床试验结果还显示临床症状改善的风险率(HR)为1.23。
论文称,尽管没有统计学显著性,但是根据预先确定的次要终点发现,与标准治疗的安慰剂组相比,接受瑞德西韦治疗的患者在出现症状后的10天内临床改善时间和有创机械通气时间缩短。
“次要终点和亚组分析不能改变研究的主要结论。”曹彬教授在论文发表后对第一财经记者表示。
曹彬教授向第一财经记者指出,这是一项设计合理的双盲、安慰剂对照、多中心随机试验。项目执行过程非常严格,极少失访。临床试验结果表明,与安慰剂对照组相比,未观察到瑞德西韦可以加快住院患者的病情恢复或降低死亡率。此外,病毒学方面,与安慰剂对照组相比,未观察到瑞德西韦可更快降低上、下呼吸道标本中的病毒载量。
基于上述结论,研究作者认为,今后的研究需要确定瑞德西韦在更早期、更高剂量或与其他抗病毒药或新冠保护性抗体联合治疗重症患者是否有效。
瑞德西韦中国临床试验设计入组重症患者453名,但最终因入组样本数量不足,试验于4月16日提前终止。
《柳叶刀》在发表瑞德西韦中国临床试验论文时,同时刊登了一篇由爱丁堡大学医疗数据主席约翰·诺里(John Norrie)教授撰写的题为《动力不足的临床研究的挑战》的评论文章。
诺里教授指出:“缺乏足够的临床试验数据的支撑意味着试验的发现不具有结论性意义。但在大流行病的背景下,数据分享尤其重要,能够快速管理相关的数据集。”
他还强调,高质量的随机试验的重要性,认为必须严格确认或者驳斥观察性数据释放出的积极信号,尤其是在疾病疗法的安全性和有效性尚未被验证的情况下。
研究终点不同导致相反结论
就在《柳叶刀》发表瑞德西韦中国临床数据的同一天,4月29日,吉利德科学公司向外释放积极信号,公布了两个好消息,一是来自美国国立卫生研究院(NIH)的临床试验已经达到主要终点,并且数据正面;二是公布了吉利德开放标签的临床试验,显示至少一半患者接受治疗5天后临床症状改善、出院,并且接受5天用药和10天用药疗效相似。除意大利外,两组患者14天死亡率都只有7%。
美国NIH对全球大约1090名患者展开的随机双盲对照临床试验显示,31%的患者在用药后症状出现改善。瑞德西韦治疗组的恢复时间为11天,对照组的恢复时间是15天;瑞德西韦组的死亡率是8%,对照组的死亡率是12%。NIAID所长福奇承认,死亡率的改善没有达到预期。
针对中美临床试验的结论为何出现截然相反的结果,曹彬教授对第一财经记者表示:“我们对用药时机有要求,时间窗口在症状出现后的12天以内,但最主要的不同是研究终点的不同,美国NIH的研究终点指标过松。如果我们使用这一指标,估计也是阴性结果。”
美国NIH和中国发表在《柳叶刀》的研究与设计均为双盲安慰剂对照临床(RCT),用药方案相同。“但是客观讲,中国瑞德西韦研究设计更加严格,科学性更强。”曹彬教授告诉第一财经记者。
从主要终点来看,美国NIH设计的指标为临床恢复时间,中国则是设计了基于6分量表的临床改善时间。“NIH的恢复定义比较宽泛,包括住院,但不需要氧疗、出院(但可能仍有活动受限、需要吸氧),相当于我们的1-2级+回家吸氧。”曹彬教授表示。
前美国FDA局长斯科特·戈特利布(Scott Gottlieb)表示:“瑞德西韦更像是新冠疾病治疗工具箱中的一部分,它不是特效药,但是如果能在患者感染的早期使用,可能会对控制疾病的发展有好处。”
新冠病毒全球确诊感染人数已经超过300万,死亡人数接近22万。在疫苗研发结果仍然需要等待相当长时间前,找到有效药物对控制疫情是至关重要的。
在这样的背景下,市场显然更愿意接受好消息。受吉利德宣布的临床试验结果提振,4月29日美股开盘后,吉利德在短暂停牌后,大涨近10%,吉利德近三个月股价涨幅超过30%。 -
#43
佩服有学者之风
-
#44
另一篇全文Gilead Sciences Statement on Positive Data Emerging From National Institute of Allergy and Infectious Diseases’ Study of Investigational Antiviral Remdesivir for COVID-19April 29, 2020 at 8:30 AM EDTPDF Version
FOSTER CITY, Calif.--(BUSINESS WIRE)--Apr. 29, 2020--Gilead Sciences. Inc. (Nasdaq: GILD) is aware of positive data emerging from the National Institute of Allergy and Infectious Diseases’ (NIAID) study of the investigational antiviral remdesivir for the treatment of COVID-19. We understand that the trial has met its primary endpoint and that NIAID will provide detailed information at an upcoming briefing.
Remdesivir is not yet licensed or approved anywhere globally and has not yet been demonstrated to be safe or effective for the treatment of COVID-19. Gilead will share additional remdesivir data from the company’s open-label Phase 3 SIMPLE trial in patients with severe COVID-19 disease shortly. This study will provide information on whether a shorter, 5-day duration of therapy may have similar efficacy and safety as the 10-day treatment course evaluated in the NIAID trial and other ongoing trials. Gilead expects data at the end of May from the second SIMPLE study evaluating the 5- and 10-day dosing durations of remdesivir in patients with moderate COVID-19 disease.
Gilead will continue to discuss with regulatory authorities the growing data set regarding remdesivir as a potential treatment for COVID-19.
About Remdesivir
Remdesivir is an investigational nucleotide analog with broad-spectrum antiviral activity both in vitro and in vivo in animal models against multiple emerging viral pathogens, including Ebola, Marburg, MERS and SARS. In vitro testing conducted by Gilead has demonstrated that remdesivir is active against the virus that causes COVID-19. The safety and efficacy of remdesivir for the treatment of COVID-19 are being evaluated in multiple ongoing Phase 3 clinical trials.
About Gilead Sciences
Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead has operations in more than 35 countries worldwide, with headquarters in Foster City, California.
Forward-Looking Statement
This statement includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors. Remdesivir is an investigational drug that has not been approved by any regulatory authority, and it has not been demonstrated to be safe or effective for any use, including for the treatment of COVID-19. There is the possibility of unfavorable results from ongoing and additional clinical trials involving remdesivir and the possibility that Gilead may be unable to complete one or more of such trials in the currently anticipated timelines or at all. Further, it is possible that Gilead may make a strategic decision to discontinue development of remdesivir or that FDA and other regulatory authorities may not approve remdesivir, and any marketing approvals, if granted, may have significant limitations on its use. As a result, remdesivir may never be successfully commercialized. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in Gilead’s periodic reports filed with the U.S. Securities and Exchange Commission, including current reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements.

-
#45
23号的报告(leaked)- 科学态度?
https://www.statnews.com/2020/04/23/data-on-gileads-remdesivir-released-by-accident-show-no-benefit-for-coronavirus-patients/
New data on Gilead’s remdesivir, released by accident, show no benefit for coronavirus patients. Company still sees reason for hope
ByEd Silverman@Pharmalot, Adam Feuerstein@adamfeuerstein, andMatthew Herper@matthewherper
April 23, 2020
The antiviral medicine remdesivir from Gilead Sciences failed to speed the improvement of patients with Covid-19 or prevent them from dying, according to results from a long-awaited clinical trial conducted in China. Gilead, however, said the data suggest a “potential benefit.”
A summary of the study results was inadvertently posted to the website of the World Health Organization and seen by STAT on Thursday, but then removed.
“A draft manuscript was provided by the authors to WHO and inadvertently posted on the website and taken down as soon as the mistake was noticed. The manuscript is now undergoing peer review and we are waiting for a final version before WHO comments on it,” said WHO spokesperson Daniela Bagozzi.
advertisement
Gilead spokesperson Amy Flood said the company believes “the post included inappropriate characterization of the study.” Because the study was stopped early because it had too few patients, she said, it cannot “enable statistically meaningful conclusions.” However, she said, “trends in the data suggest a potential benefit for remdesivir, particularly among patients treated early in disease.”
The data (for details, see screenshot below) will be closely scrutinized but are also likely imperfect. The study was terminated prematurely, which could have affected the results. The context that would be provided by a full manuscript is missing, and the data have not been reviewed as normally occurs before publication.
advertisement
Related: The coronavirus pandemic could shrink some biotech startup valuations
Many studies are being run to test remdesivir, and this one will not be the final word. Results are expected soon from a Gilead-run study in severe Covid-19 patients, although that study may be difficult to interpret because the drug is not compared to patients receiving only standard treatment. Encouraging data from patients in that study at the University of Chicago were described by researchers at a virtual town hall and obtained by STAT last week. However, unlike those data, these new results are from a randomized controlled trial, the medical gold standard.
Gilead is also running a study with a control group in more moderate Covid-19 patients, and the National Institute of Allergy and Infectious Diseases is running a study that compares remdesivir to placebo. There are even more studies of the drug ongoing.
According to the summary of the China study, remdesivir was “not associated with a difference in time to clinical improvement” compared to a standard of care control. After one month, it appeared 13.9% of the remdesivir patients had died compared to 12.8% of patients in the control arm. The difference was not statistically significant.
“In this study of hospitalized adult patients with severe COVID-19 that was terminated prematurely, remdesivir was not associated with clinical or virological benefits,” the summary states. The study was terminated prematurely because it was difficult to enroll patients in China, where the number of Covid-19 cases was decreasing.
An outside researcher said that the results mean that any benefit from remdesivir is likely to be small.
“If there is no benefit to remdesivir in a study this size, this suggests that the overall benefit of remdesivir in this population with advanced infection is likely to be small in the larger Gilead trial,” said Andrew Hill, senior visiting research fellow at Liverpool University.
He added that the results of the study should be pooled with larger studies being conducted by Gilead using a technique called meta-analysis to allow for “a balanced view of the efficacy of remdesivir from all randomized trials.”
-
#46
各个高人都是牛人说的头头是道,也不知道有没有道理。
作为投资者,我只相信资本市场。你们慢慢聊,我买我的股票就对了。 -
#47
同意你完全赞同,还是希望能出更多特效药,无论中西
-
#48
股票和科学态度是两回事股票更接近于政治 - 呵呵呵
钱要赚,科学态度也要严谨 - 哈哈哈哈 -
#49
这也是正面的表述,领导都喜欢这样的而实际结果就是 5天比10天更好
准确的表达就是 -这个药用5天就好了, 不需要用10天, 如果用5天还治不好,就安天命吧
是不是这样?
科学道理和依据呢?
从什么时候开始给药,然后算5天, 确诊? 或者有明确症状? 比如SPO2<90(95%)....
都没有提到
这个只是新闻稿, 不是研究报告, 继续等! -
#50
Gilead成功了 -原来真的是政治完全是为了平衡这篇文章-人家发在柳叶刀《the Lancet》
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext